IPPCAAS Unveils New Mechanism of Phosphorylation and Ubiquitination Synergistically Regulating the Homeostasis of OsRbohB to Modulate Rice Broad-Spectrum Resistance
Recently, the Crop Pathogen Functional Genomics Research Innovation Team at the IPPCAAS, published a research paper titled “Phosphorylation and ubiquitination synergistically promote the degradation of OsRbohB to modulate rice immunity” in the top-tier plant biology journal The Plant Cell. This study systematically elucidates the molecular mechanism by which the stability of the OsRbohB protein is precisely regulated by phosphorylation and ubiquitination, thereby modulating broad-spectrum resistance (BSR) in rice. This provides a new strategy for crop disease resistance breeding.
Crop varieties with BSR are beneficial to combat pathogens infection as they can offer resistance against a wide range of pathogen races or multiple pathogen species. During plant-microbe interactions, reactive oxygen species (ROS) serve as key signaling molecules involved in immune signal transduction, and NADPH oxidases, which belong to the respiratory burst oxidase homolog (Rboh) family, are responsible for ROS production. Given the critical role of ROS homeostasis in balancing plant immunity and growth, how to enhance BSR by regulating Rboh protein remain unresolved.
This study demonstrated that overexpression of OsRbohB significantly enhances rice resistance against both Magnaporthe oryzae (rice blast) and Xanthomonas oryzae pv. oryzae (bacterial blight). Mechanistically, the calcium-dependent protein kinase OsCPK4 directly interacts with the N-terminus of OsRbohB, regulating its protein stability by phosphorylating serine sites at 322 and 326 (Ser322/Ser326). Interestingly, phosphomimic OsRbohB weakened the immune response, suggesting that phosphorylation may act as a negative regulator of immune signaling. Moreover, after screening E3 cDNA library, the RING-type E3 ubiquitin ligase OsRING142 interacts with and ubiquitinates OsRbohB at Lys266 for degradation by the 26S proteasome pathway and compromising the immune response. Remarkably, phosphorylation at OsRbohB facilitates OsRING142-mediated ubiquitination and degradation of OsRbohB. Both modification sites simultaneously mutated in rice exhibited stronger disease resistance, demonstrating that this synergistic mechanism is a central hub for immune balance.
This research not only highlights the critical role of Rbohs in broad-spectrum resistance but also demonstrates that N-terminal phosphorylation and ubiquitination together constitute a precise brake for the immune response, attenuating ROS bursts in a timely manner to balance plant growth and immune defense. The findings provide a new target and material for genetic improvement of crop disease resistance, and offer a new perspective for deciphering the regulatory network of plant immune homeostasis.
Dr. Hui Tao from the IPPCAAS is the first author, and Professor Yuese Ning is the corresponding author. This study was supported by grants from the National Key Research and Development Program of China and the National Natural Science Foundation of China, Innovation Program of the Chinese Academy of Agricultural Sciences.
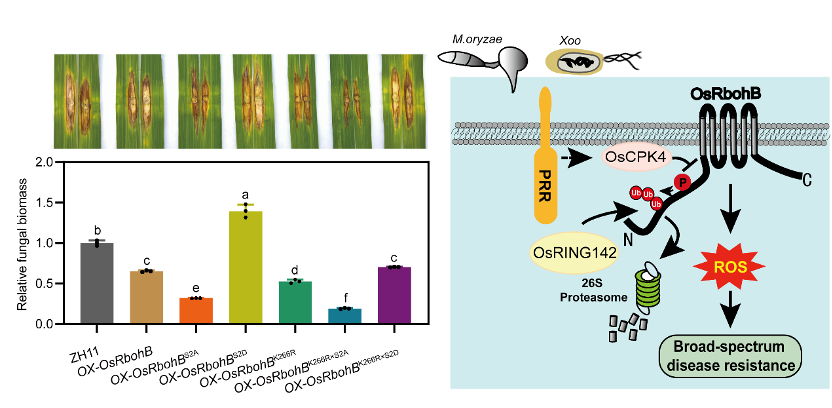
Figure. OsCPK4 Phosphorylation and OsRING142 Ubiquitination Synergistically Regulate OsRbohB
-
 International Symposium on Plant Biosafety (ISPB 2025) Convenes in Guangzhou — Science-led plant health governance to secure food systems and advance the SDGs
International Symposium on Plant Biosafety (ISPB 2025) Convenes in Guangzhou — Science-led plant health governance to secure food systems and advance the SDGs -
 Three decades of China's membership of CABI celebrated at 2nd International Symposium on Plant Biosafety
Three decades of China's membership of CABI celebrated at 2nd International Symposium on Plant Biosafety -
 CABI receives recognition from FAO for its work to support sustainable plant production and protection
CABI receives recognition from FAO for its work to support sustainable plant production and protection -
 Second Round Notice | International Symposium on Plant Biosafety (ISPB 2025)
Second Round Notice | International Symposium on Plant Biosafety (ISPB 2025)
